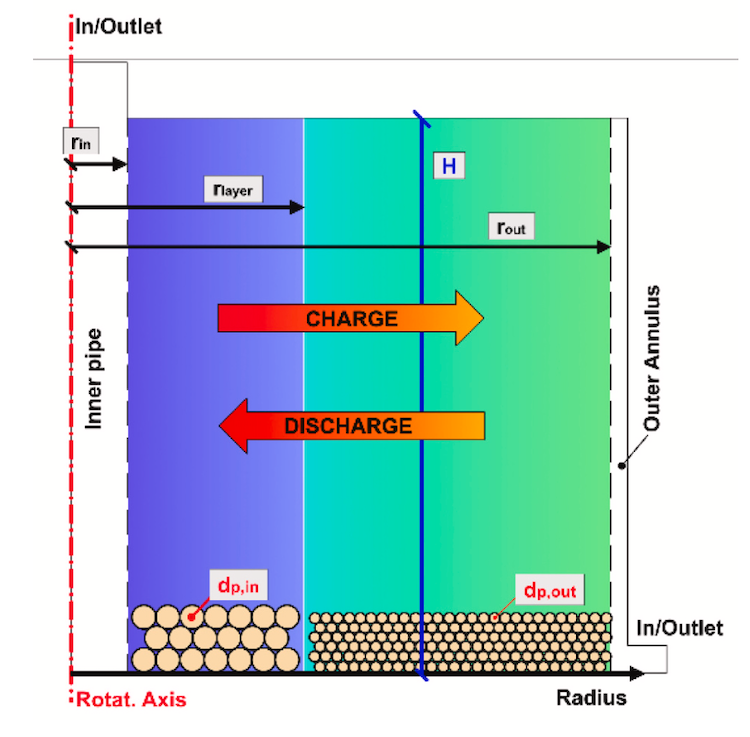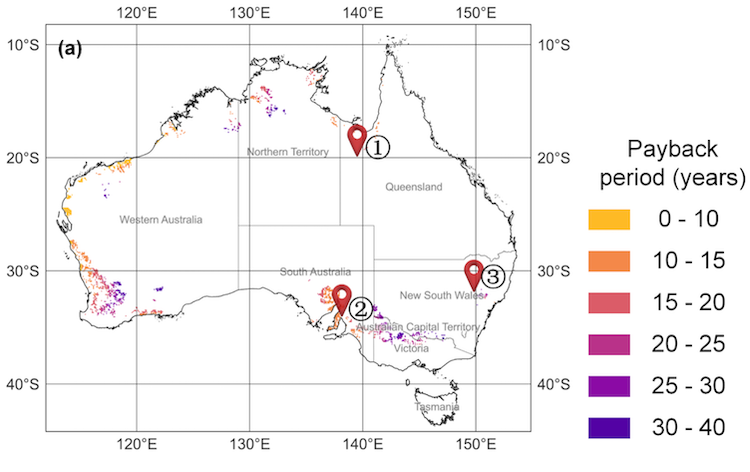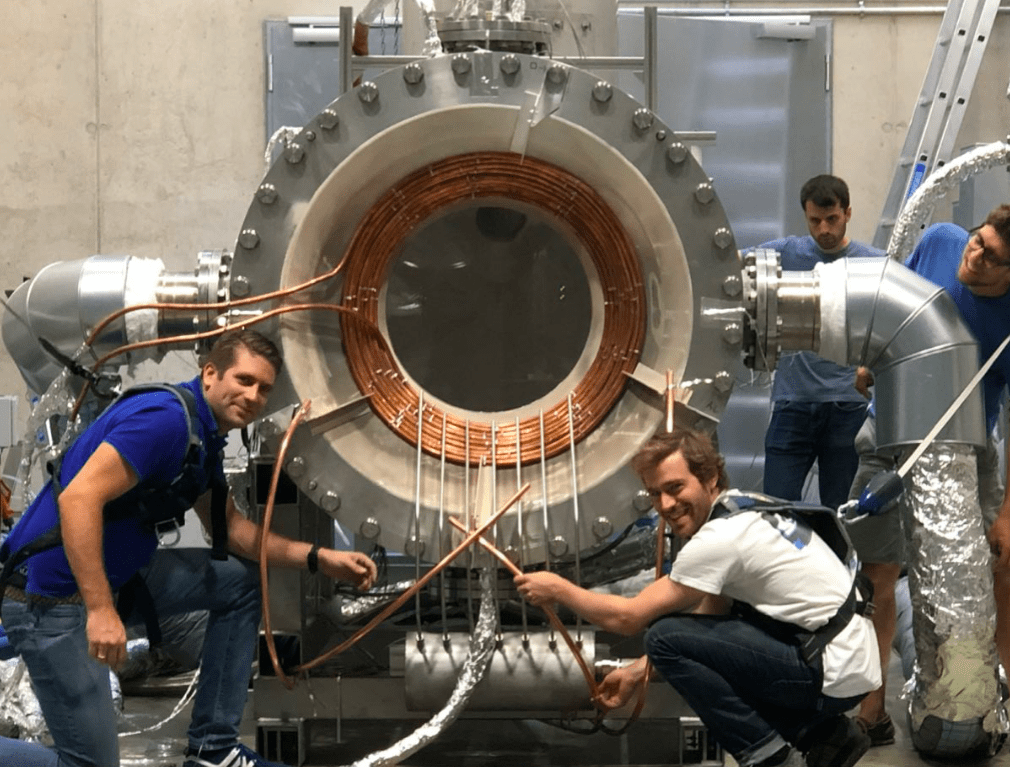
IMAGE@Synhelion Solar researchers Philipp Furler (left) and team working on the small scale 200 kW Synhelion solar receiver pilot at DLR’s Synlight solar research facility in Jülich, Germany. This demo successfully completed first tests, reaching temperatures in excess of 1’500 °C.
The Swiss startup Synhelion is working on commercializing two routes to solar jet fuel with the help of Italy’s Eni, the 10th-largest oil company in the world.
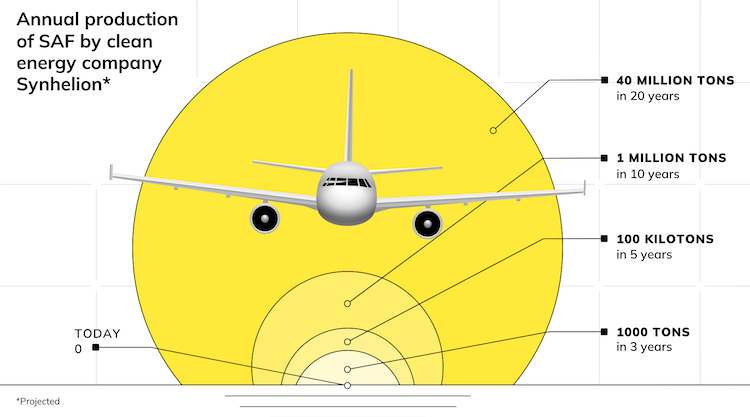
The plan is to market aviation fuel in two stages; first, by 2022 to sell a solar jet fuel based on solar reforming of methane, with 50% lower carbon emissions. This will fund development for sale by 2030 of a more advanced product; 100% carbon-free solar jet fuel from air capture of CO2 and H2O, which the research teams have demonstrated successfully.
“The pure water and CO2 splitting process we proved is our long-term vision, but it still requires quite some development and its product is more expensive than the current fossil fuel price, so it is currently too far away from the market. In this more advanced pure thermochemical route we are putting in 1500°C of heat to drive the chemical process. We aim to introduce this technology by 2030,” explained Synhelion CTO Philipp Furler, whose solar fuels research at ETH Zurich formed the basis of the technology.
“But we also plan to introduce a product before that time. In order to enter the market much faster we want to launch a simpler solar reforming-based route first. The solar reforming approach is more efficient than the pure water and carbon dioxide splitting process and based on existing industrial technology. This is why we are developing both processes in parallel.”
Goal; jet fuel from air by 2030
Synhelion’s long-term 100% solar fuel technology developed out of solar fuels research at ETH Zurich in Switzerland to manufacture jet fuel (kerosene) from carbon dioxide and water captured from air, and is based on solar thermochemistry using heat as the energy input for producing the syngas, which is subsequently converted into liquid fuels.
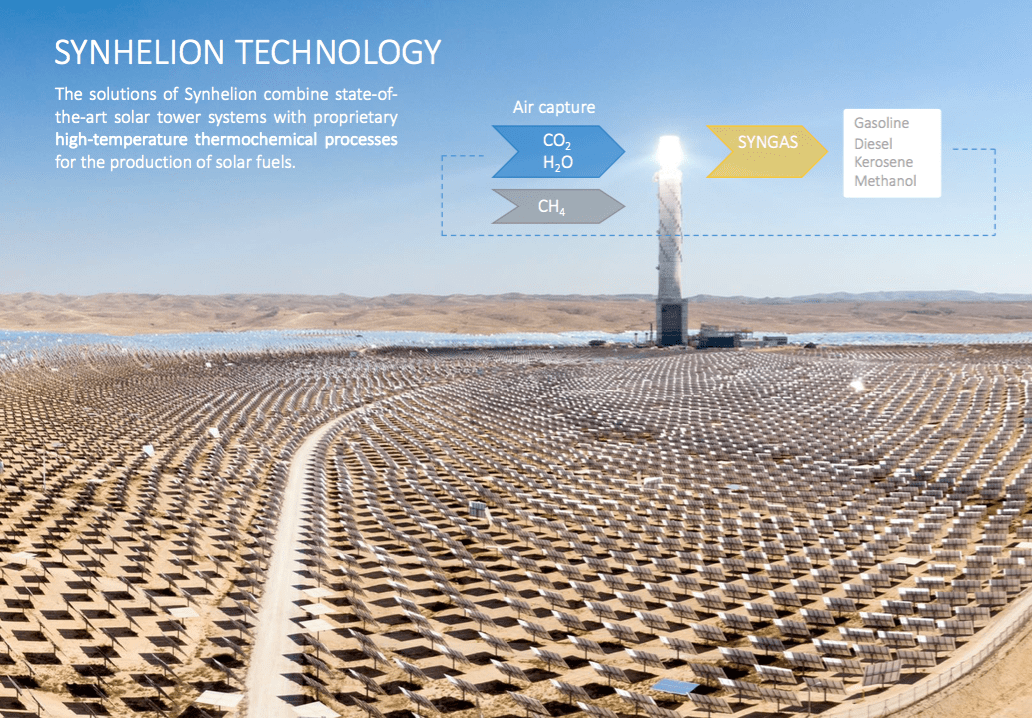
IMAGE@Synhelion – A solar field of mirrors and tower for solar jet fuel production would look like today’s tower CSP projects
SolarPACES previously interviewed Furler on the 100% solar jet fuel from air when the team had progressed the technology to a record-breaking solar reactor efficiency of 5%, from just 1% five years earlier. Based on the testing, Furler believes Synhelion can reach beyond the 20% reactor efficiency needed to make the jet fuel reasonably economical. But larger scale is needed to test.
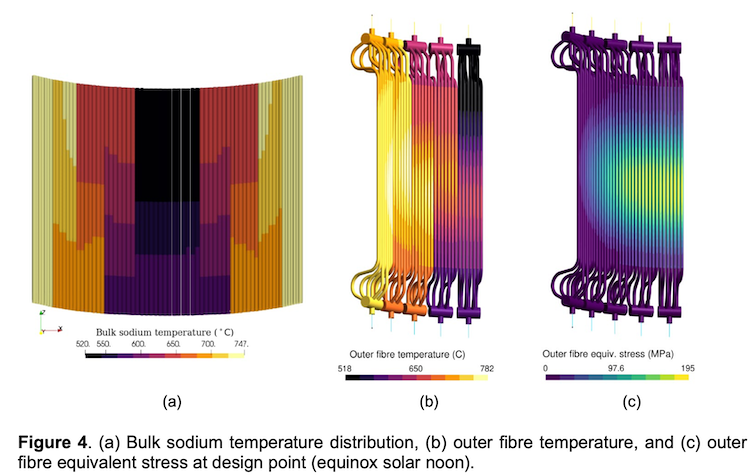
“We developed a novel concept which allows sensible heat recovery in the process to boost the efficiency beyond 20%,” he said. “Our tests showed that our prototype systems operate successfully, but we haven’t reached higher efficiencies yet, because they need to be built in bigger dimensions. If you operate thermal storage it needs to be at a large scale. Otherwise, heat losses through the sidewalls dominate the results.”
To get accurate test results and high efficiencies; building at large scale is the key. The start-up plans to scale-up the technology step-by-step to industrial size over several years. This time is required to thoroughly solve the engineering challenges that arise by increasing the size and lowering the cost of the technology.
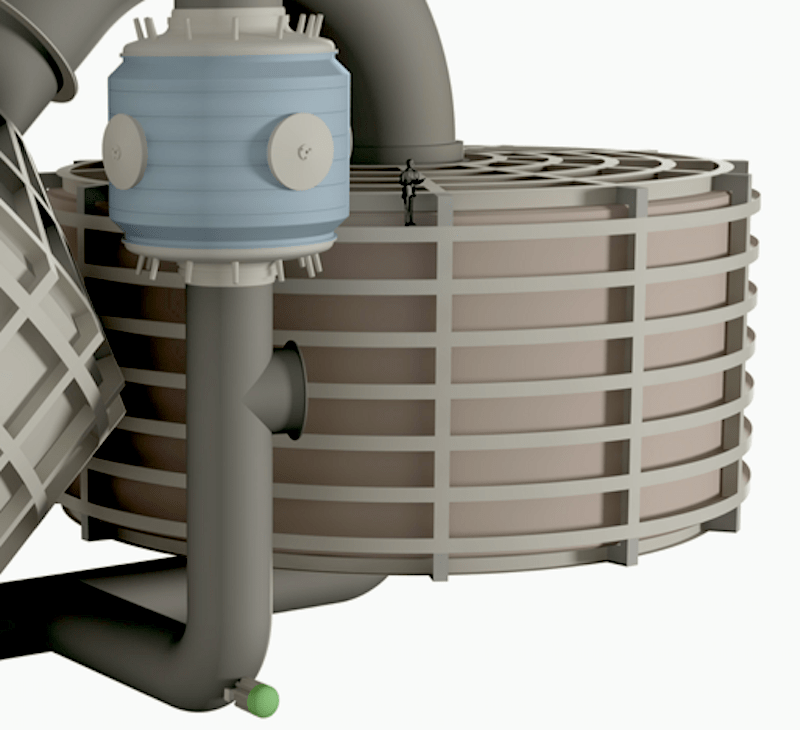
IMAGE@Synhelion A man pictured on the storage tank, shows the scale needed. The solar receiver partially shown at left receives solar heat from the solar field, the reactor, center, uses that solar heat to rearrange H2O and CO2 into syngas, and the storage, right, ensures that the reactor always has the heat it needs to run around the clock.
The goal is to enter the market with 100% solar jet fuel from air by 2030. In parallel, the solar reforming fuels would be commercialized by 2022 so that they can start generating revenues.
First to market; solar reforming
Jet fuel made through a solar reforming-based approach would be commercially viable in the short term, and cost little more than today’s jet fuel because reforming is a mature, standard technology.
Solar reforming of a methane source uses the same technology as current reforming, except heat from a solar receiver is substituted instead of heat from burning natural gas. This is a well understood, comparatively low temperature process.
“For solar reforming the temperatures are between 800°C and 1100°C. This process is substantially easier for us, and it is already implemented in the industry. Solar reforming research started many years ago by several groups, and there is a great deal of potential in this pathway. We can produce a fuel which has about 50% lower CO2 emissions at a similar price to fossil fuels. This allows us to address large markets in the next couple of years. We can count on a mature industrial technology to a certain extent and this makes the development much faster,” explained Furler.
“For the methane source we could also use any biogas like landfill gas and produce 100% CO2-neutral fuels. However, we assume that for very large-scale production we are going to need natural gas because there is not enough biogas available.”
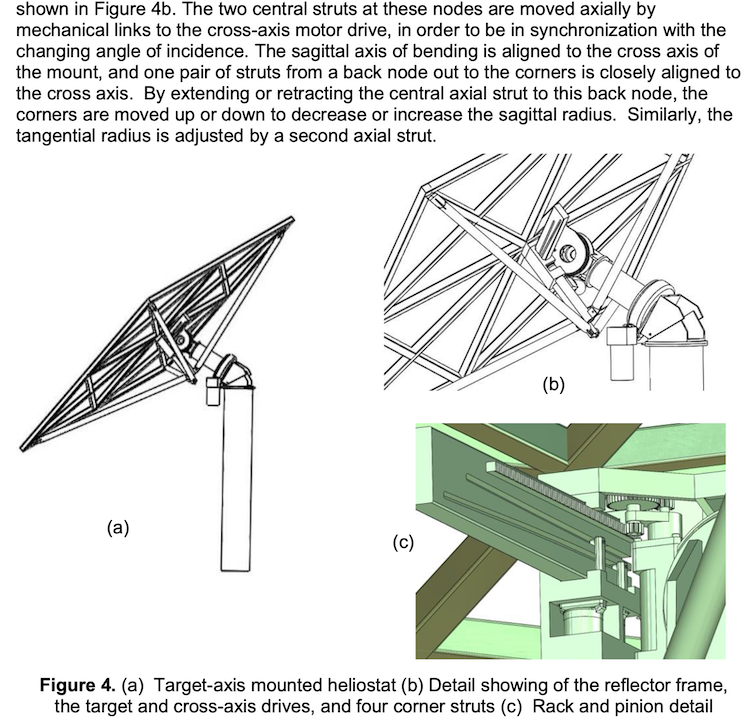
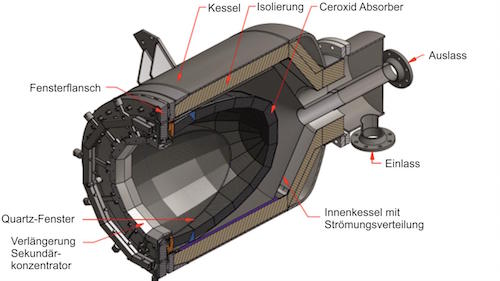
The two technologies have a lot of overlap. Both operations are performed in the same infrastructure pictured: receiver, reactor and storage

IMAGE@Synhelion: Tower schematic for Synhelion solar jet fuel production
How the processes work
Both technologies have essentially three steps; Step one; capture the ingredients. Step two; rearrange them into syngas. Step three; convert syngas into kerosene; aviation fuel (or other liquid hydrocarbons).
Solar reforming at 800°C -1100°C
For the solar reforming route, for step one, they can get CO2 and water either from a point source like methane or from direct air capture. Synhelion will perform step two, delivering 800°C to 1100°C of heat from a solar field of mirrors to the reactor to perform the thermochemistry to produce syngas. The syngas will then be converted to liquid fuels, via standard industrial technology, such as Fischer-Tropsch synthesis, to produce the commercial aviation fuel.
Solar jet fuel from air at 1,500°C
For the jet fuel from air route; for step one, Synhelion is partnering with a company such as ETH Zurich spinoff Climeworks that captures CO2 and H2O directly from air. Synhelion will perform step two, delivering 1,500°C of heat from a solar field of mirrors to the reactor to perform the thermochemistry to turn these hydrocarbons into syngas. And again in step three, the syngas is converted to commercial aviation fuel, the same way as it is done today by large oil and gas companies like Eni.
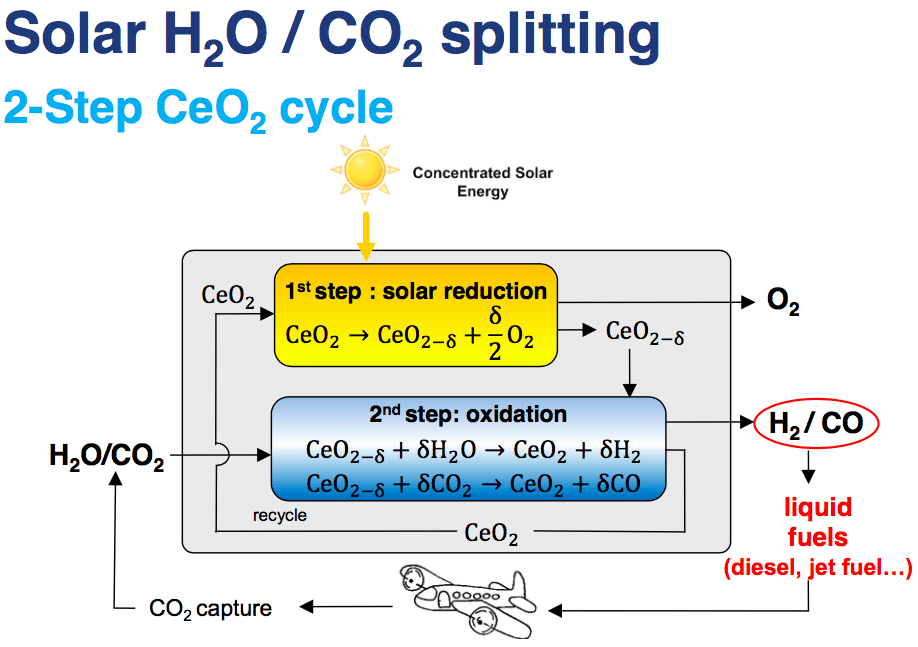
IMAGE@ Philipp Furler Diagram of the jet fuel from air process
With more than 10 patents filed (and more coming) and longtime solar fuels research experts Furler and Aldo Steinfeld on board, the Synhelion start-up could potentially be a real solar fuels success story that is solidly grounded in great science.
Selected publications attached:
Hoes 2019: Additive-Manufactured Ordered Porous Structures Made of Ceria for Concentrating Solar Applications
Marie Hoes, Simon Ackermann, David Theiler, Philipp Furler, and Aldo Steinfeld
Dählera 2018 Optical design and experimental characterization of a solar concentrating dish system for fuel production Fabian Dählera, Michael Wilda, Remo Schäppia, Philipp Hauetera, Thomas Coopera, Philipp Gooda, Carlos Larreaa, Max Schmitza, Philipp Furler, Aldo Steinfeld
Geissbühlera 2018: GeissbÅhler2018-An assessment of thermocline-control methods for packed-bed thermal- energy storage in CSP plants, Part 1: Method descriptions
L. Geissbühlera, A. Mathurb, A. Mularczyka, A. Haselbachera,
Marxer 2017: Solar thermochemical splitting of CO2 into separate streams of CO and O2 with high selectivity, stability, conversion, and efficiency Daniel Marxer, Philipp Furler, Michael Takacs and Aldo Steinfeld
Furler 2014: Thermochemical CO2 splitting via redox cycling of ceria reticulated foam structures with dual-scale porosities Philipp Furler, Jonathan Scheffe, Daniel Marxer, Michal Gorbar, Alexander Bonk, Ulrich Vogt and Aldo Steinfeld
Furler 2012: Solar thermochemical CO2 splitting utilizing a reticulated porous ceria redox systemPhilipp Furler, Jonathan Scheffe, Michal Gorbar, Louis Moes, Ulrich Vogt,and Aldo Steinfeld
Furler 2012: Furler2012 – Syngas production by simultaneous splitting of H2O and CO2via ceria redox reactions in a high-temper Philipp Furler,a Jonathan R. Scheffea and Aldo Steinfeld*abFurler 2012:
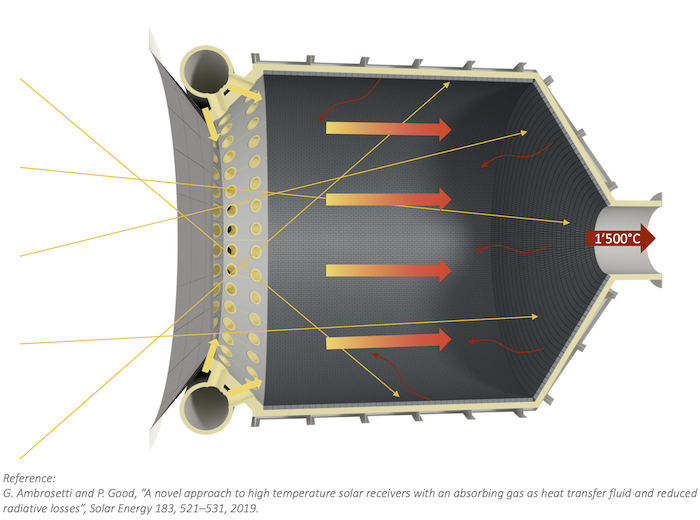
Ambrosetti 2010: A novel approach to high temperature solar receivers with an absorbing gas as heat transfer fluid and reduced radiative losses Gianluca Ambrosetti, Philipp Good
Chueh 2010: High-flux solar-driven thermochemical dissociation of CO2 and H2O using nonstoichiometric ceria William C. Chueh, Christoph Falter, Mandy Abbott, Danien Scipio, Philipp Furler, Sossina M. Haile, Aldo Steinfeld