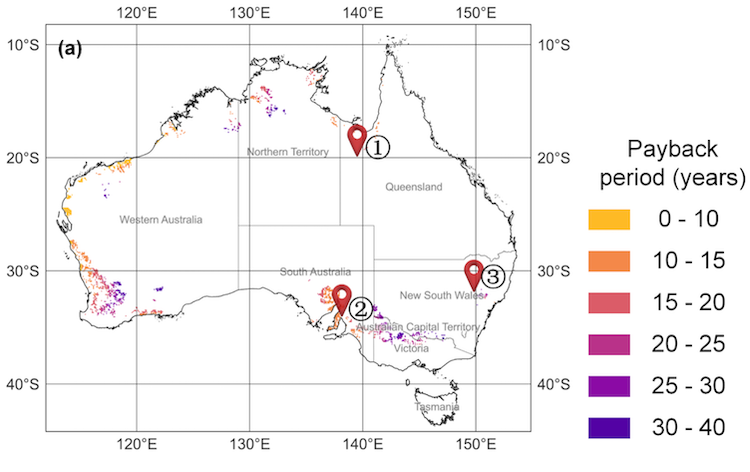
After the solar heat trap experiment measuring the temperature increase to 1050°C at the back of the solar receiver inside a solid block of quartz, even at the front, the temperature measured 450°C IMAGE©Solar thermal trapping at 1,000C and above.
Solar receiver efficiency in concentrated solar thermal (CST) energy is determined by how much solar radiation can be converted into useful heat for industry processes or solar thermochemistry. Heat lost to outside air reduces efficiency, so researchers are looking for ways to better “trap” the heat inside the solar receiver.
Now, an ETH team has, for the first time, demonstrated that solar heat can be effectively trapped in a solid block of semi-transparent quartz at a temperature above 1,000°C. They published the results in the journal Device: Solar thermal trapping at 1,000C and above.
While previous studies have proposed using thermal trapping to improve efficiency, previous literature focused on lower temperatures. In the last decades, many researchers at ETH in Switzerland, CNRES in France, DLR in Germany, Weizemann in Israel and at Sandia in the US have proposed to exploit thermal trapping at higher temperatures. However, this work presents the first results dedicated to precisely characterizing the temperature inside the heat trap to demonstrate this phenomenon at above 1,000°C.
“The idea came to me after reading some old papers from the 1960s where they proposed the thermal trap concept for the first time,” lead author Emiliano Casati told SolarPACES in a call from Switzerland.
“Back then, they investigated the concept at a low temperature. What I wanted to do was to demonstrate that thermal trapping works at high temperatures, too.”
The original research was developed for flat plate collectors decades before today’s commercial solar receivers atop a central tower and heated by mirrors. But for tomorrow’s world, heat-trapping will enable higher temperatures with fewer heliostats (mirrors). So the ETH research helps advance CST for decarbonizing industries like cement and metal manufacturing.

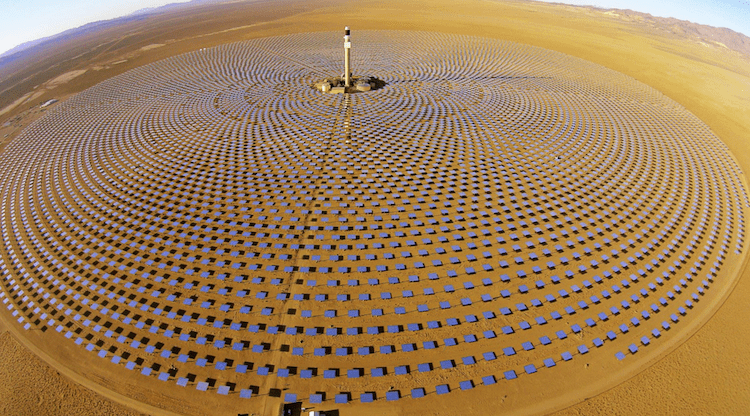
Tower CSP In the foreground and Trough CSP behind it in this photo of the NOOR I,II,II CSP project at Ouarzazate. Today’s commercial solar receivers atop a central tower are heated by a solar field of mirrors.
How heat trapping works
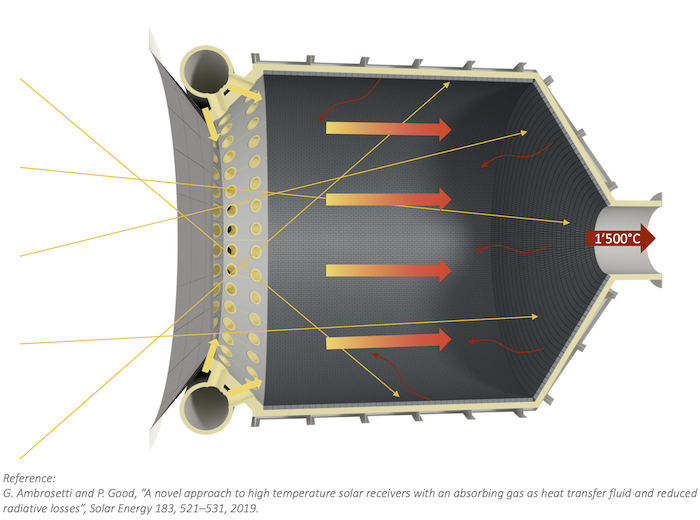
When sunlight hits opaque materials like metals, it gets absorbed at the surface and conducted through the material, so the surface it hits is the hottest part.
However, some semi-transparent materials, including quartz, methane, water, and CO2, absorb more infrared radiation. This energy penetrates the interior and is absorbed inside rather than heating just the surface.
So with the right semi-transparent material in a solar receiver, sunlight could heat the inside of the receiver hotter than the front, trapping the heat deep inside the receiver where it is needed, rather than staying at the surface where it risks leaking back out to the outside air. The efficiency of these systems depends on how much sunlight they can turn into useful heat.
After the team ruled out some other proposed materials for their demonstration, like semi-transparent aerogels, which could not hold as high a temperature, they chose a solid semi-transparent block of synthetic quartz, from Heraeus Conamic (Heraeus Suprasil CG), which can hold heat up to over 1000°C.

For the solar heat trap measurement the researchers used a solid rod of quartz. IMAGE©Solar thermal trapping at 1,000C and above.
Pros and cons of quartz for the heat trap test
The solid quartz was chosen for its availability and well characterized properties, plus its thermal stability at the target temperatures.
“At high temperatures, the calculation, the simulation of radiative heat transfer is much more complex,” Casati explained.
“The light goes in, but most of it can’t get back out. Everything changes because the material itself starts to emit radiation as well. Experiments are also more complex, especially temperature measurements.”
“So it’s really we demonstrated the phenomenon at high temperatures. Now, if others want to investigate different materials, they can build on our work. But I don’t see quartz receivers coming to market because quartz is expensive and difficult to work with,” Casati noted.
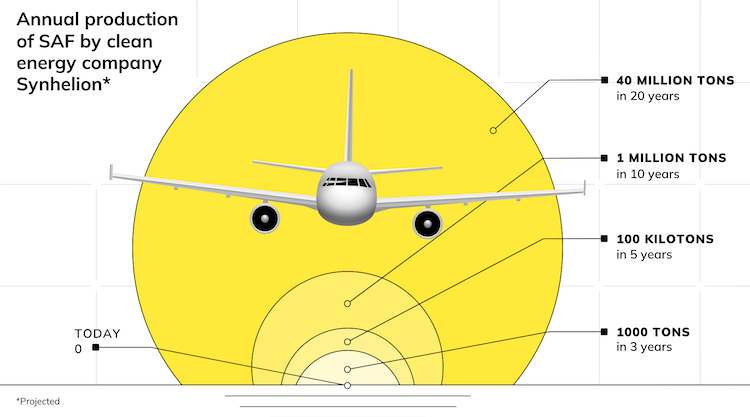
Some other contenders for absorbing infrared radiation in solar receivers are greenhouse gases like carbon dioxide, methane, and water vapor. The ETH spinoff Synhelion has harnessed the heat-trapping effect of steam and CO2 in its very high-temperature solar receiver to make aviation fuel with solar heat, for example.
How the demonstration worked
For the demonstration of the phenomenon, the lab scale test was at under 1 kilowatt. For their experimental setup, the ETH team exposed a 30 cm (11”) cylindrical quartz rod to concentrated solar radiation at ETH’s high-flux solar simulator. They aimed the simulated sunlight at the exposed circular surface at the front of the solid rod of quartz that contacted an opaque silicon carbide disk at the back. The tests used a concentration of about 135 suns, or135 kW/m2 of radiative flux at the setup aperture.
”Here, we demonstrate this effect at high temperatures with a quartz rod attached to an opaque absorber plate reaching 1,050°C when exposed to 135 suns of concentration, while the quartz front face remains at 450°C.“ the paper states.
The rod’s walls were coated with platinum to create specular surfaces and lined with insulation to minimize any boundary effects on the heat transfer. To measure the gradations of temperature from the front to the back of the receiver, thermocouples were placed at points along the quartz rod.
”The core of the work was to measure the temperature inside the material. And doing that at a very high temperature in a semi-transparent medium is not trivial,” Casati commented.

The difference between a receiver with and without a heat trap is shown. The inclusion of the heat trap, shown in blue on the right increases the temperature as it travels inside the solar receiver, and delivers the higher temperature from the absorber. IMAGE©Solar thermal trapping at 1,000C and above.
The heat trap results
Temperatures measured along the rod’s axis showed that while the absorber at the back reached temperatures around 1,050°C, the front surface of the semi-transparent material only reached around 450°C, demonstrating the heat-trapping effect.
“So the heat internally at the absorber can go down into the thermal storage or the reactor or whatever it is that somebody wants that heat for,” he noted.
About 90% of the incoming radiation reached the absorber, with only about 6% absorbed by the quartz over its length. The measured temperatures closely matched their modeled results, differing by under 5%.
Their results were proven repeatable over eleven experimental runs, each lasting up to 8 hours. During these runs, heating continued until reaching a stable 1,050°C, varying by less than a degree over at least 10 minutes.
The paper suggested that this high thermal efficiency can be even further refined by minimizing any sunlight reflection at the surface by using coatings or geometric patterning.
What this means
Normally, it takes a much bigger solar field with thousands of heliostats to achieve a higher concentration. If the heat can be trapped better in the solar receiver, it reduces heat losses, increasing efficiency, and makes it possible for fewer heliostats and smaller, more economical solar fields. This is important for the new application of concentrated solar heat for industrial processes, where typically, there’s limited space for large solar fields of mirrors.
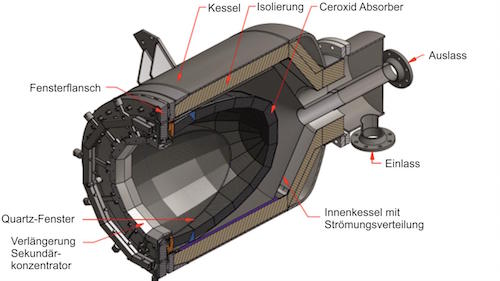
How can Synhelion’s solar receiver achieve such high temperatures?