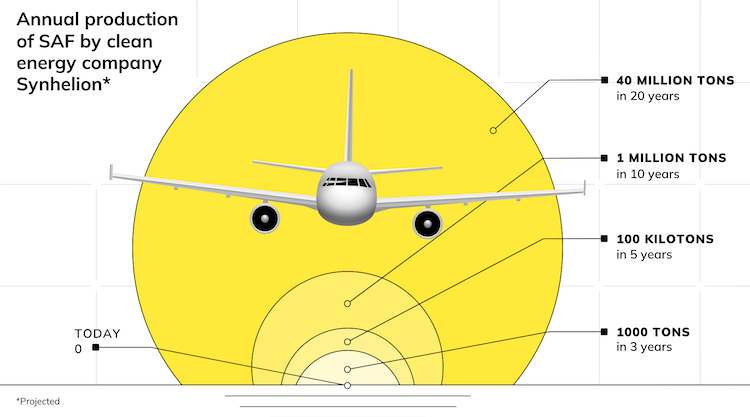
How a breakthrough solar thermochemistry process that uses direct solar heat to cycle between sulphur and sulphuric acid would generate “virtually unlimited” seasonal thermal energy storage

Sulfur can be stored like a pile of coal. “This cycle allows you to get energy out of the sulphur and store it in between. Why it’s in focus now is that we can use 100% renewable energy – concentrated solar – to heat the reaction. That’s why chemical companies now come in and are interested in demonstrating the plant.”
Researchers are now refining a groundbreaking long-duration thermal energy storage technology in the SUPHURREAL project
Molten salts are currently state-of-the-art for solar thermal energy storage. But elemental sulphur has more than an order of magnitude greater energy storage capacity, and is ideally suited to seasonal thermal energy storage, DLR Institute of Future Fuels research head Christian Sattler noted in a call from Germany.
“Sulphur’s energy density is so much higher than that of molten salt, and also you get high-value heat,” he explained. “You have a very cheap storage medium in the sulphur, and since we propose chemical combustion, you can adjust the temperature to whatever temperature level you need.”
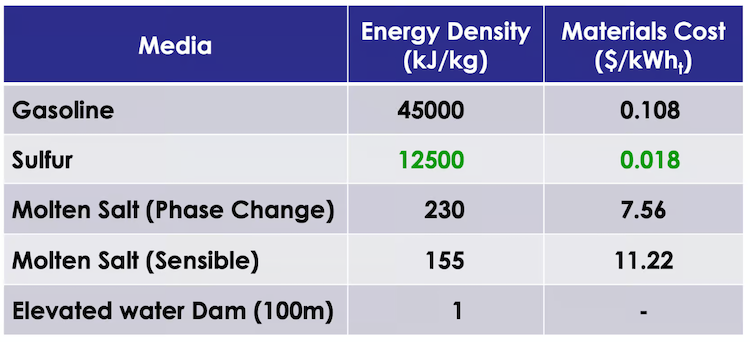
Sulphur energy density / cost compared ©Allothermally Heated Reactors for Solar-Powered Implementation of Sulphur-Based Thermochemical Cycles
Most thermal energy storage materials aren’t combusted when used for heat. Sulphur is different. Like a pile of coal, sulphur would be stored in a pile outside, and then, like coal, it is burned when the heat is needed.
But there’s a huge difference between burning coal and burning sulphur. Imagine if when you burnt a lump of coal, a new lump of coal was created. In a solar thermochemical sulphur cycle that Sattler’s team has been working on, when you combust the sulphur, you would generate new sulphur to burn again…
How the solar thermochemical cycle makes sulphur renewable
Each time you burn sulphur, it creates sulphur dioxide that can be processed to sulphuric acid, which can be reacted to create sulphur again. So this continuing cycle lends itself to a thermochemical process that endlessly cycles between sulphuric acid and sulphur.
“The sulphur can either be reused in the storage cycle to close the loop, or it can be further processed to produce a surplus of sulphuric acid as a valuable commodity,” Sattler said.
“This cycle allows you to get energy out of the sulphur and store it in between. Why it’s in focus now is that we can use 100% renewable energy – concentrated solar – to heat the reaction. That’s why chemical companies now come in and are interested in demonstrating the plant.”
From CORDIS EU Research Results: Sulphur poised to transform the future of solar energy storage
“During this cycle, the sulfur is collected to form a pile, and the H2SO4 is stored in suitable tanks. When the sun is shining, the sulfur pile grows while the H2SO4 tank is emptied. During the night or when it is cloudy, the sulfur pile reduces [as some is burned, generating H2SO4] so the H2SO4 tank fills up”:
And then, because sulphur can be stored in a pile in ambient air, and is not affected by rain, sulphur is just as available as coal was, but with no CO2 emissions, and renewable, due to this thermochemical cycle.
Why a sulphur thermochemical cycle is relevant now: Solar heat
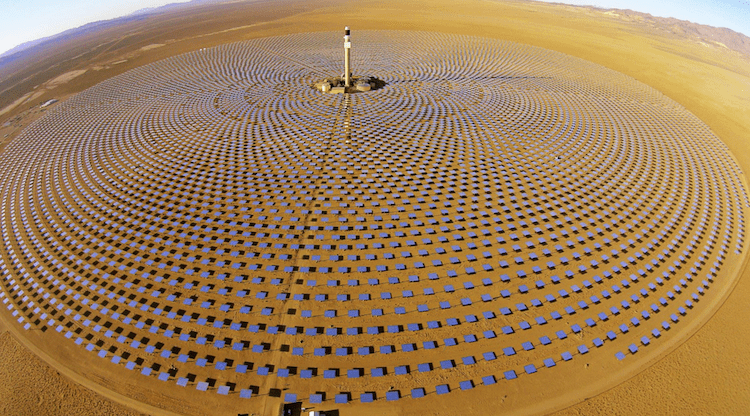
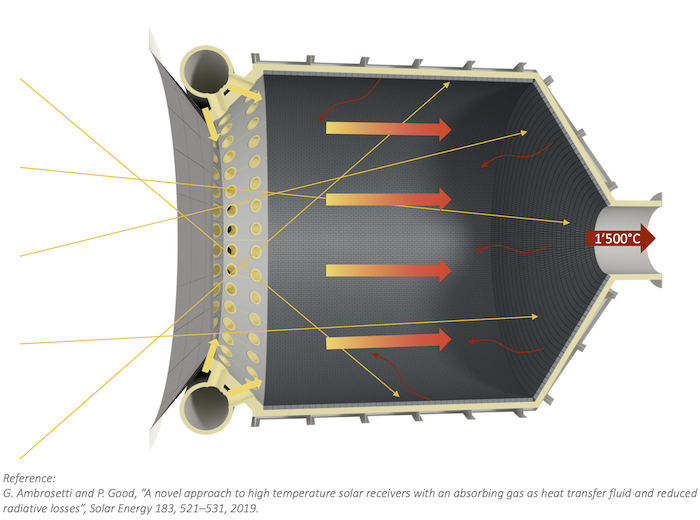
Today’s concentrated solar thermal can generate very high-temperature heat from its solar field of heliostats. Particle receivers like DLR’s CentRec® can translate the reflected solar flux into temperatures over 1000°C. Synhelion’s gas-based receiver gets even hotter; 1500°C. This means concentrated solar thermal can now run many thermochemistry processes that used to require the burning of fossil fuel.
The first heating step of the sulphur cycle uses heat in the 450-500°C range when sulphuric acid is dissociated into steam and sulphur trioxide (SO3). The second step uses between 700-950°C for catalytic dissociation to sulphur dioxide (SO2) and oxygen.
Sulphur cycles were already known. In response to the oil shocks of the 1970s, General Atomics had proposed using the hybrid sulphur cycle but tapping nuclear power rather than concentrated solar for the heat, to generate hydrogen and sulphuric acid. However, that idea was shelved once the fossil fuel industry regained hegemony, and since that time, hydrogen has been produced from steam reforming of natural gas.
But in today’s more climate-conscious world, green production of hydrogen is back in focus, along with the resulting need for renewable energy to provide seasonal energy storage to guarantee continuous chemical processes like this solar sulphur storage cycle.
Sulphur’s advantages (for both green hydrogen and seasonal storage) are its low cost, high energy density, wide availability and that it’s easily shipped by truck, rail or ship (liquid or powder). The challenges of the sulphuric acid step are mitigated as they are well-known in the industry. The harmful emissions that caused acid rain in the 20th century are no longer a risk (and this sulphur cycle is in an enclosed loop). The management of corrosive sulphuric acid has an even longer history of over 100 years of containment. And sulphuric acid is widely available.
Today, the fossil industry generates the most sulphuric acid, but in our post-fossil future, chemicals and mining would supply the feedstock.
“The whole chemical industry is available everywhere, so you can start a cycle with the sulphuric acid,” Sattler pointed out. “Although you could start the cycle at any point. It doesn’t have an end, and it doesn’t have a beginning.”
The copper industry, a major industrial source of sulphuric acid to start the cycle, is an ideal partner to grow the use of renewable sulphur heat as a replacement for fossil heat. (This team also works on a related solar sulphur cycle for hydrogen production using sulphuric acid from the copper industry)
Because copper is already an essential metal for its superior electrical conductivity, used in wiring, wind turbines, batteries, inverters, and electric motors. It will only become even more critical as the move to “electrify everything” replaces fossil fuel for power with solar panels, wind power, and electric vehicles.
Next steps in this solar sulphur cycle for seasonal energy storage
By 2021, under the PEGASUS project, Sattler’s team at DLR, along with KIT and several European partner companies had already demonstrated first-of-its-kind sulphuric acid splitting for thermal energy storage. They demonstrated it in simulated solar energy indoors in the 300 kW reactor at Synlight, which is heated by the equivalent of a 10,000 “suns” solar field of heliostats. Their papers include Allothermally Heated Reactors for Solar-Powered Implementation of Sulphur-Based Thermochemical Cycles.
Now with funding under the current SULPHURREAL project, “aimed at demonstrating and validating a breakthrough approach for next generation, carbon-free, direct conversion of solar energy into chemicals storable for a virtually unlimited time” these teams have begun the next step, refining novel reactor designs and methods of applying and distributing the catalysts within the reactors.
“The SULPHURREAL project is targeted on the one hand to develop disruptive catalytic technologies for the two catalytic steps of this solid sulphur thermochemical cycle, namely the high- (800-850 C) and medium- (600-650 C) temperature catalytic SO3 splitting to SO2 and oxygen and the subsequent disproportionation of SO2 to solid sulphur and sulphuric acid…
SULPHURREAL will further develop and upscale a first-of-its-kind sulphur burner operating at power density > 5 MW/ m³ at ambient pressure and having demonstrated potential for prolonged operation at power densities of > 75 MW/cbm for typical operating pressure of 15 bar by simulations.”
Commercial readiness
In two years, the team is due to demonstrate their progress at DLR’s outdoors 1 MW CentRec® solar receiver “on-sun.”
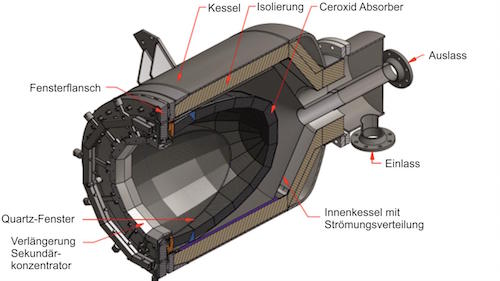
DLR’s particle receiver uses centrifugal force to create a dense particle film that directly absorbs concentrated solar radiation. Bauxite or sand particles remain stable without degrading at much higher temperatures than molten salts, enabling high-temperature thermochemistry operations.Thousands of mirrors aim concentrated sunlight up to the receiver, and the heat is then transferred in particles to heat the reaction chamber.
Some of the process steps, like the receiver, are already ready for operational demonstration now (the CentRec® has already been licensed to Heliogen and others for commercial use), but the team will further validate aspects of the solar sulphur generation process during the next two years.
“The industrial interest reflects that the process has good chances,” Sattler noted.
“So we want to find out with our main industrial partner, Grillo Werke AG, what is necessary to deploy it and what that will cost.”
New Sulfur-Based Solar Reactor Makes Cheap Green Hydrogen for Copper Mines