Hybrid-Sulfur (HyS) solar reactor
By producing solar hydrogen as an industrial service for copper mining, the scale is established for mass production of green hydrogen at much lower cost than electrolysis.
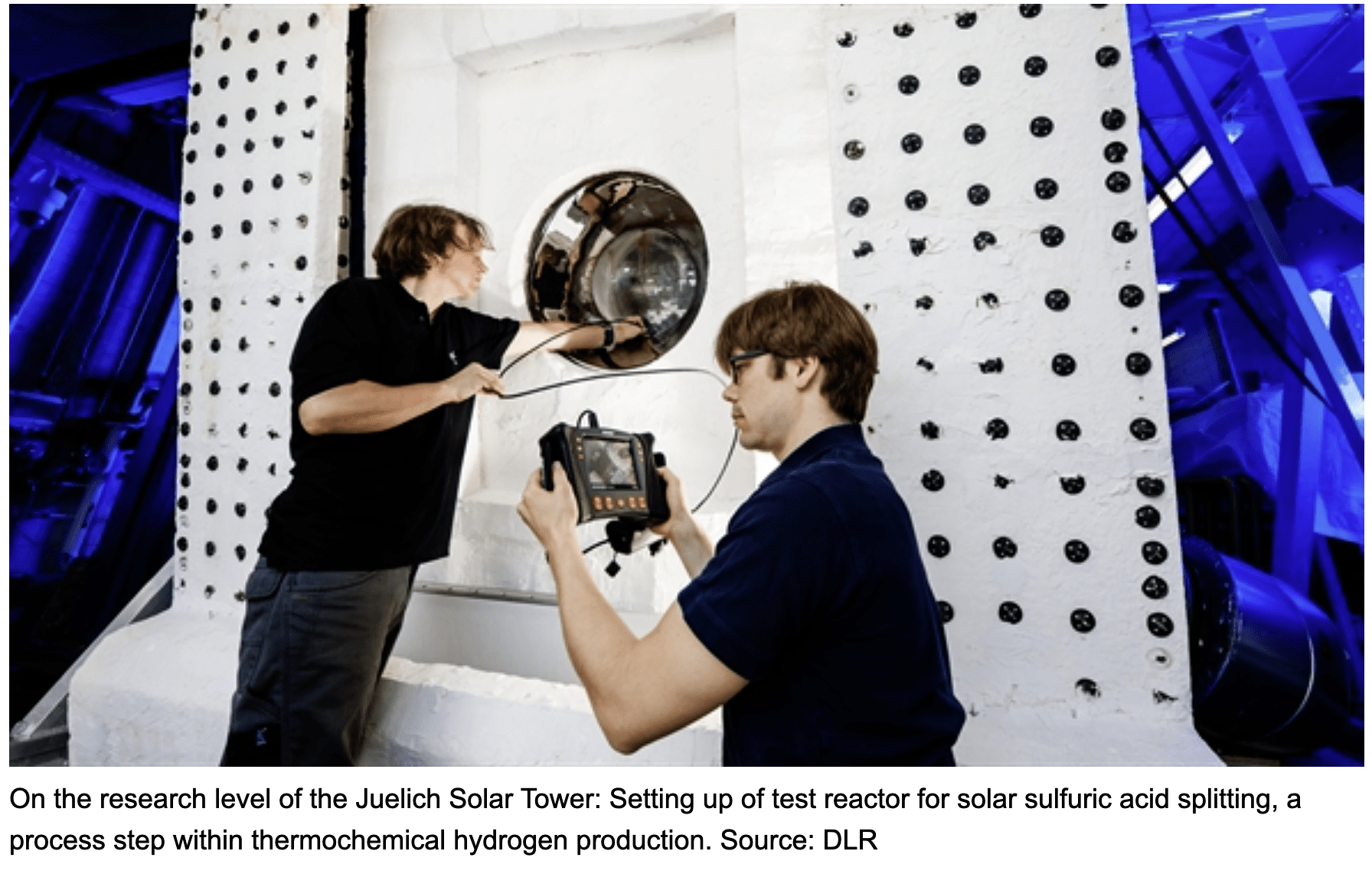
The fastest route to commercializing solar hydrogen could be by supplying hydrogen, oxygen and heat for Australia’s copper mining industry with solar thermochemistry, Christian Sattler told SolarPACES recently. As in all solar fuels production, the heat for this chemistry is produced by mirrors aiming concentrated solar flux to a receiver atop a tower.
With his experience heading solar fuels research at Germany’s solar research center at DLR, Sattler sees a synergistic connection between the sulfuric acid processing used in copper mining with a new solar way to make hydrogen from sulfuric acid; the Hybrid-Sulfur (HyS) technology.
How is sulfuric acid used in copper mines?
Sulfuric acid reactions are used to refine copper from ore using hydrogen and oxygen.
“There are mining processes like copper ore roasting where sulfur dioxide is produced. This can be used in a hybrid thermal/electrical cycle to produce hydrogen, oxygen and sulfuric acid,” Sattler explained.
“Copper mines need both hydrogen and oxygen. They need the oxygen first to roast the ore because there’s a lot of sulfur in it. With the roasting, you transform the ore into copper oxide and then you need hydrogen to reduce the copper oxide to copper. The copper ore contains the sulfur you use to produce the sulfuric acid which in turn you use to leach out the other impurities in the minerals.”
Extracting hydrogen from sulfuric acid with solar uses much less energy than water electrolysis
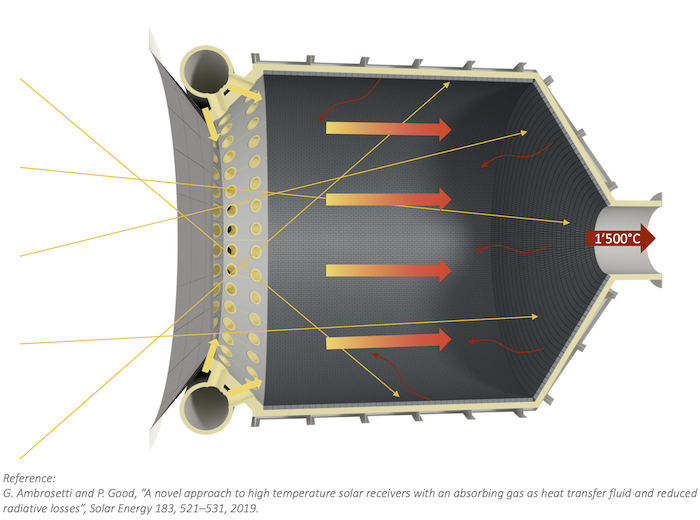
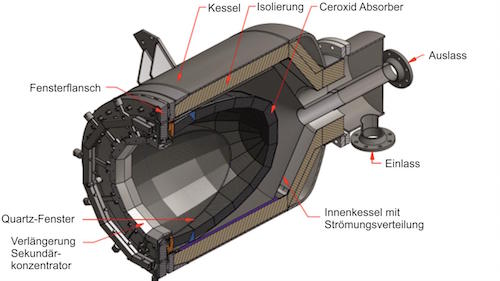
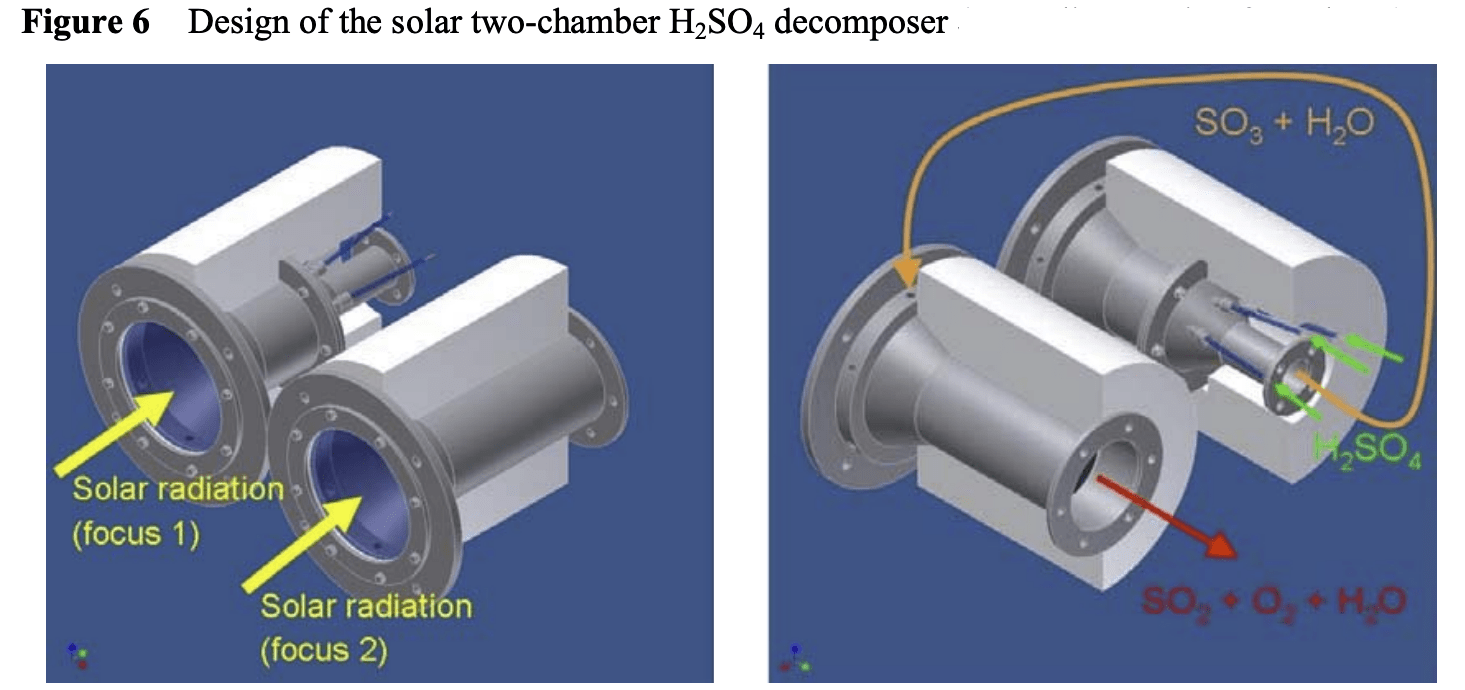
Using sulfuric acid, both hydrogen and oxygen can be generated in a new kind of solar reactor. In the two-step HyS process, high temperature solar heat at up to 900 °C is used to decompose sulfuric acid (H2SO4) to generate hydrogen and oxygen.
The decomposition of sulfuric acid is part of the first process step of producing sulfur trioxide (SO2) and oxygen. In the second step, the SO2 together with water goes to an SO2-depolarised electrolyzer (SDE) to generate hydrogen and fresh H2SO4, to repeat the cycle.
This SO2-depolarised electrolyzer only requires about a seventh of the electrical energy of conventional water electrolysis, so this method can produce about 50% more hydrogen with the same solar input.
International research groups in Germany, Australia, Japan and the US have engineered and tested the component parts needed for this very high temperature sulfur-based process – a solar reactor/evaporator, a heat exchanger for SO3 decomposition, and an oxygen separator for SO2 and O2. Materials search identified construction from silicon carbide which can maintain stability in the corrosive conditions and the very high temperatures needed to perform the thermochemistry.
Many advantages of sulfur-derived solar hydrogen
Sulfur can be stored cheaply for a long time just in a pile outdoors; like a pile of coal. Unlike all other heat storage technologies, its stored energy can be retrieved at a temperature actually higher than that of the original heat input, and recovered at a constant temperature.
A sulfur-based thermochemical process has more than an order of magnitude greater storage capacity than today’s molten salts, which would cut the cost of reliable round-the-clock operation of solar fuels production.

Win-win: make solar hydrogen and oxygen onsite at the copper mine
Currently, because of the expense of getting hydrogen and oxygen to the mines and the cost of fossil fuels for heat for the chemical reactions needed, copper mines only produce copper oxide on site, and then they have to send the copper oxide off to another company for refining into copper, so the mines lose out on producing the valuable end product of copper.
“This HyS technology will be able to provide that on site. So if you are able to produce hydrogen and oxygen at the same site where the copper mine is – you will be independent of an external company, that otherwise has to produce the gases elsewhere to refine the copper oxide. This increases your costs.” Sattler explained.
“We’ve designed a system that we can integrate the HyS into copper processing,” added Mehdi Jafarian, who is contributing to HyS research at the School of Mechanical Engineering at Australia’s University of Adelaide which will trial the new technology, first at lab scale, and outdoors on-sun in 2022.
“So we are working with DLR for the integration of the HyS into copper processing to produce hydrogen and oxygen in situ for the copper processing and mining sector. This enables us to supply copper processors with hydrogen and oxygen for like nine to ten months a year. In economic terms it is even comparable with the state-of-the-art hydrogen production technologies using fossil fuels.”
Once solar hydrogen is produced as part of an industrial service to the copper mining industry, the scale is established for its mass production as a standalone energy carrier with many applications in hard-to-decarbonize sectors, such as cargo shipping.