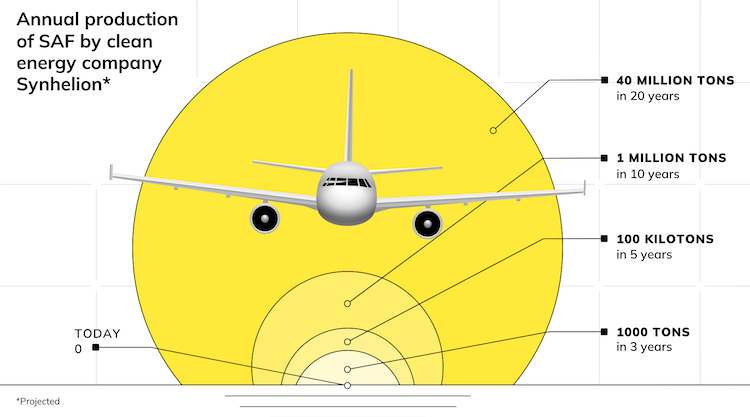
High-temperature molten salt degradation at over 620°C gets an oxygen fix that also solves tank corrosion as well
The molten salt mixture deployed in today’s commercial CSP plants for thermal energy storage is liquid at 565°C and degrades at higher temperatures. Yet higher temperatures would increase thermal efficiency. So how can we develop a molten salt mix that can reach temperatures up to 620°C without degradation? And at the same time, how can we prevent such a high-temperature molten salt liquid from corroding tanks and piping.
An interesting approach is being pursued by a team of 15 researchers at the German Aerospace Center (DLR) led by Thomas Bauer, with funding from the German Federal Ministry for Economic Affairs and Climate Action (BMWK) under the VeNiTe and Limelisa projects.
A fix that kills two birds with one stone
A single solution actually solves both challenges, according to DLR researcher Dr. Alexander Bonk, who leads a sub-team on the topic.
“Yes, the two go hand in hand,” he affirmed in a call from Germany.
“This high-temperature chemistry we are assessing is not only about the molten salt’s thermal stability, but also about the chemistry of the containing materials or piping materials that leads to corrosion. And this topic is still quite new even for us. So we were starting corrosion experiments above 600°C two years ago.”
His group is performing lab bench scale experiments focused on the chemistry involved. He explained that the solution lies not so much in the molten salt liquid alone – but in a synergistic relationship between the composition of two elements. Between the fluid itself – and the airspace in the contained system.
Ambient air chemistry affects the molten salt chemistry
Bonk explained that the molten salts release extra oxygen atoms that outgas into the air in the tank.
“So you go from your initial molten salt compound, which contains nitrogen and three oxygen atoms (nitrate ion), to something that only contains two (nitrite ion), which means you’re losing oxygen in this reaction step,” he said.
“And this oxygen is not going to a random place, it’s diffusing into the gas phase. If a molten salt tank is operating at 565°C you will form 4% to 5% of nitrites in the molten salt system and oxygen is released to the environment. As long as you have oxygen present in the gas phase, your nitrite will also react back to the nitrate, which means you will form a chemical equilibrium. At higher operation temperature the nitrite ion can further decompose into corrosive oxide ions, accompanied by the formation of other gas species.”
This equilibrium holds steady at current temperatures as long as all reagents are retained in the system. Nonetheless, these gases that form in the tank can only react back into the liquid if the tank is entirely airtight with no exposure to ambient air outside. However, in state-of-the-art operation, excess gases produced in the reaction will unavoidably get flushed out into ambient air, usually undergoing a catalytic treatment.
Molten salt degradation and corrosion at higher temperatures
At present commercial temperatures, the loss of reactive gases to the atmosphere is a minor issue. However, at elevated temperatures above 565°C, the potential formation of corrosive ions in the molten salt requires more careful consideration.
“Which means if you want to go to these high temperatures, you need to think about gas management in general,” he said.
“If you know that these gases form at high temperatures, you can try to stabilize the molten salt by re-introducing the gases into the system. For us, that means, for example, if we operate molten salts in a gas phase containing more oxygen and other reactive gases, the molten salt will be stabilized. And this is something we take advantage of in our experiments in lab- and technical scale. From the chemical side, depending on temperature, there’s going to be an optimum reactive gas concentration that needs to be present in the tank.”
Bonk believes his team is on course to avoid these twin problems; degradation in the molten salt mix and corrosion of the tanks at higher temperatures. But he apologized for being unable to give more precise detail than is in their conference paper Thermal Energy Storage using Solar Salt at 620 °C- How a reactive gas atmosphere mitigates corrosion of structural materials. This is because his team has a confidential agreement with industry partners interested in these higher temperatures for thermal energy storage.
Goal: Develop molten salt thermal storage for industries like coal plants and process heat applications
Their research aims to include standalone thermal energy storage in Germany’s coal fleet. The idea is to gradually “flexibilize” coal power plants using surplus electricity from the grid stored thermally onsite and the heat used by the existing thermal power plant assets in the power block.
“You’d try to build the storage unit into the existing facility, and then replace your coal by increasing your storage, to more or less dynamically transition from coal to renewable energy so you can later use PV and wind to run the same steam cycle without the coal,” he explained.
Instead of using heat from burning coal to boil water for steam, these coal plants would be run on stored thermal energy heated by electricity from the grid. The temperature their research is focused on is 620 °C.
“That’s because here in Germany, some of our modern coal-fired power plants have a steam temperature which cannot be covered with state-of-the-art 565 °C molten salt technology,” Bonk pointed out.
“And if you want to run this steam cycle from a molten salt storage system, then it needs a slightly higher temperature between your storage medium and steam cycle. We think there’s a realistic goal of increasing the temperature to something around 620 °C with a few tricks to integrate this technology, for example, into coal-fired power plants.”
This higher temperature grid-charged thermal storage would also be helpful to industries that now burn fossil fuel for process heat at or under 600°C. One example is the provision of superheated steam for chemical sites for combined heat and power generation. Furthermore, existing concentrating solar thermal power plants can benefit from the development to better control the salt chemistry in the power plants and thus increase the lifetime.