Ammonia (NH3) is key to enabling a solar hydrogen (H2) future, says a prominent Australian researcher.
Solar energy could be stored, bottled and shipped globally in existing ammonia infrastructure as a zero carbon liquid fuel, according to Keith Lovegrove, the author or co-author of over 170 research papers and technical reports.
His comments come at a time when ARENA, the Australian Renewable Energy Agency has just announced $20 million in funding for Renewable Hydrogen For Export.
Lovegrove contributed to Australia’s Roadmap to SolarFuels comparing various solar and hybrid fuels to replace coal as a new cleaner energy export economy for Australia. Japan already accounts for a third of Australian energy exports, and is investing in a hydrogen economy.
“Japan is a major industrialized country completely dependent on energy imports. They are suggesting that their future involves moving to a hydrogen economy. Australia is a pretty good place to make hydrogen,” he said in a Skype call this week.
But he said that a molecule that actually packs in more hydrogen than hydrogen (H2), holds the greatest potential to unleash a clean hydrogen economy. Ammonia (NH3) bonds together 1 nitrogen atom but 3 hydrogen atoms. “Amazingly enough, there’s a greater mass of hydrogen in a liter of liquid ammonia than there is in a liter of liquid hydrogen,” Lovegrove explained. “It’s counterintuitive, but ammonia is just a better molecule at packing together with itself.”
Perhaps, instead of the future H2 economy, we should really be planning for ‘the NH3 economy’ he suggested. Like hydrogen, with no carbon atoms, ammonia makes an ideal clean liquid fuel, but one with an easier transition.
“I’m personally in favor of using ammonia to transport hydrogen,” he said. “You can then turn it into hydrogen when you get there. Or you can even use it directly, because with adapted conventional gas turbines, you can burn ammonia directly.”
Ammonia as potential energy storage for CSP
Lovegrove formerly represented Australia for IEA’s SolarPACES Task II: Solar Chemistry Research and now heads the solar thermal division of energy consultancy ITPower. His recent research, in collaboration with partners at UCLA includes a 2017 paper at Chemical Engineering Progress, Leveraging the Ammonia Industry for Solar Energy Storage – investigating NH3 as a novel energy storage material for concentrated solar power (CSP).
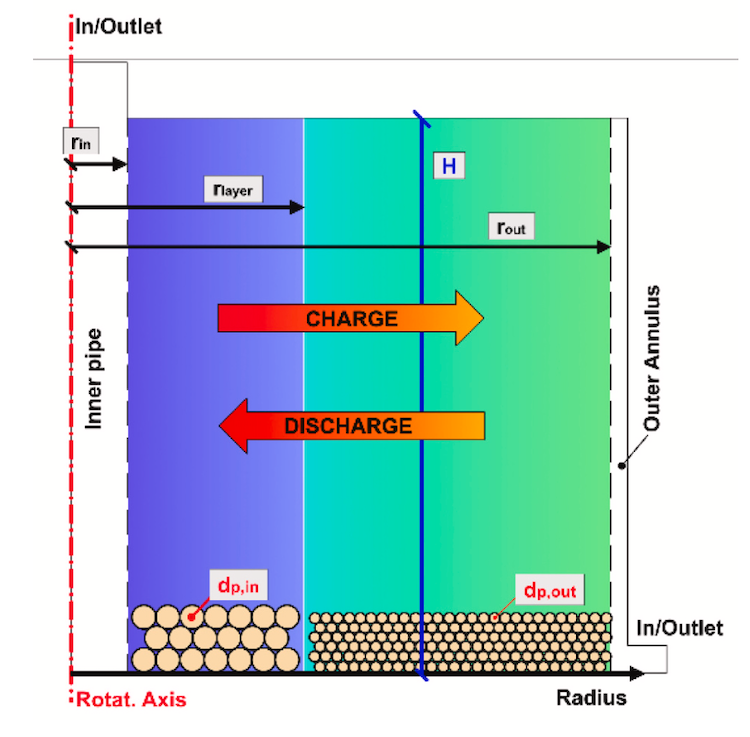
As storage, NH3 is decomposed and later recombined in a repeating thermochemical energy storage cycle. While the reactions take place at around 700°C for storing energy and releasing it later for steam, the hydrogen and ammonia gas mix would be delivered and stored at ‘room temperature’ and only create heat when needed to power a steam cycle, so it can be stored for long periods till needed.
But Lovegrove sees NH3 as useful far beyond CSP storage.
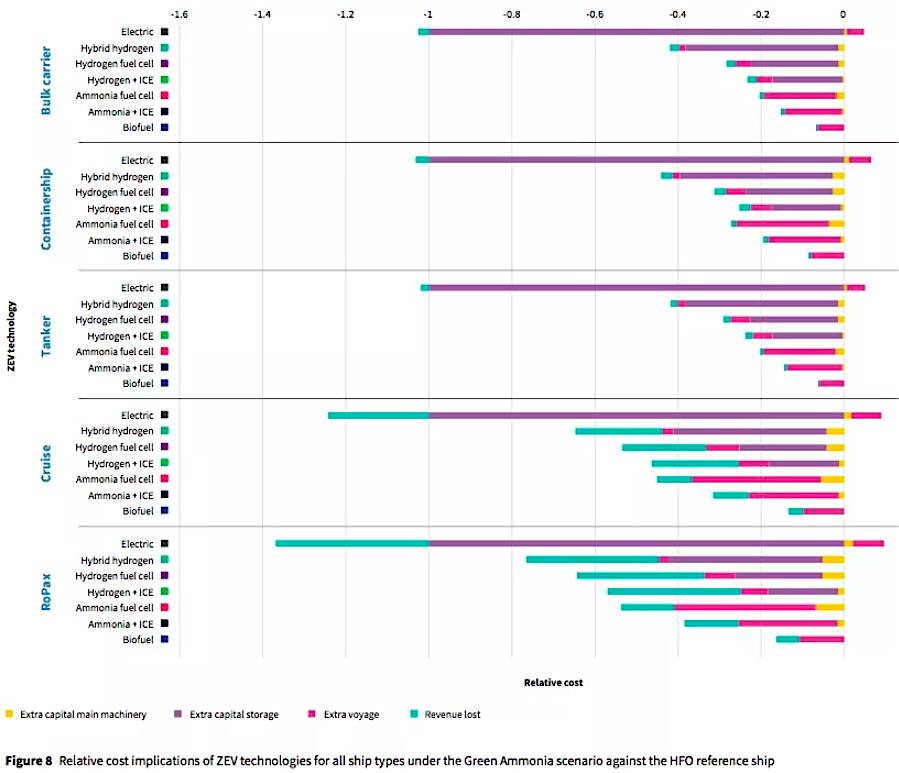
IMAGE CREDIT: Lloyd’s Register (ammonia is also the cheapest (sustainable) clean fuel to actually power shipping itself)
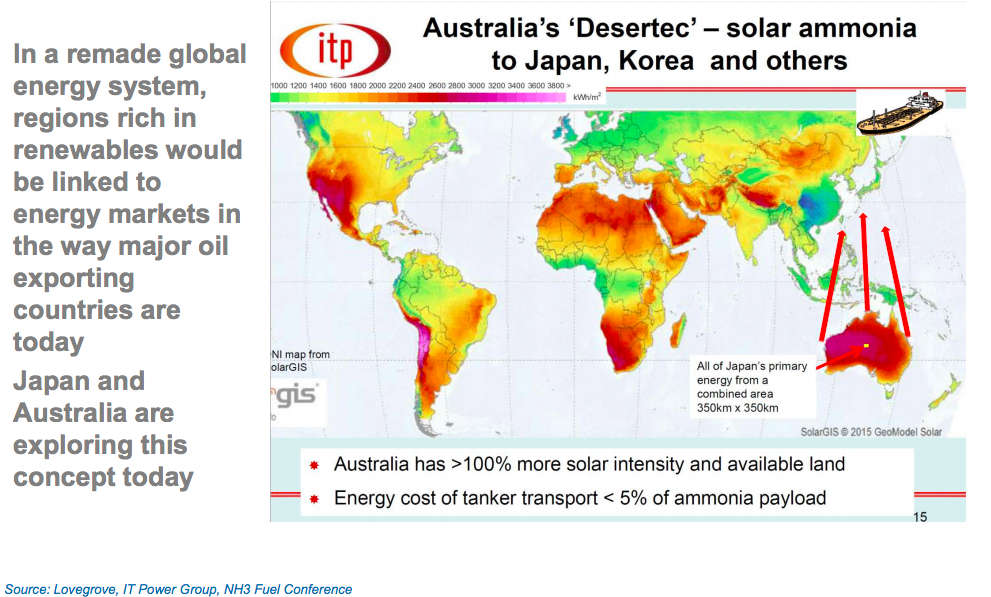
Shipping solar by sea opens the world’s deserts to CSP
In densely populated regions, it is difficult to site utility-scale CSP, which needs particulate-free air found in quiet deserts, yet also a nearby population that needs electricity, a combination not found everywhere. But, if instead of transmitting electricity on wires, ammonia can ‘bottle’ solar in a liquid fuel that can be sent and used anywhere, the market grows considerably.
Solar could be produced in a desert, sent to the coast in pipelines and shipped globally. Solar exports in ammonia would supercharge the production and export of solar thermal energy anywhere in the world because sea transport of liquid fuel is extremely efficient.
“Take as a precedent the trade in oil products now. If you fill a tanker up with oil in the Middle East or wherever and you go halfway around the planet you burn up an amount of energy doing that which represents about 2% of its payload,” he calculated. “So tanker transport is 98% efficient. Then the interesting question is how to similarly carry hydrogen.”
H2 and NH3 can both store and carry energy, but ammonia is more ideal as an energy carrier, he said. “Ammonia production for fertilizer is one of the world’s biggest chemical industries. There’s plants all over the world, and ships moving it about on a daily basis, so it’s a very standard thing.”
Because it is already widely produced and used, with well established distribution and handling procedures, it wouldn’t be a difficult transition. When ammonia is burnt as a fuel it simply returns to nitrogen and water. If it leaks into the air, it is easily detected by smell.
Why liquid fuels matter for climate
Liquid fuels are the largest untapped source for decarbonization. Even if all the electricity in the world was 100% renewable, the entire electricity sector currently accounts for only 20% of global emissions.
Thirty years of trying has not brought the solar hydrogen economy to fruition, mostly because hydrogen entails new infrastructure to transport and store it. Researchers are increasingly looking at ammonia, with shipping and containing infrastructure in place, as a potential carrier.
Ammonia can not only store and ship renewable energy, it can also be made using any renewable source of electricity.
Because Ammonia is simply hydrogen and nitrogen, it can be split chemically from just water (H2O) and air (73% nitrogen) using electricity. Any chemical reaction is basically the exchange of electrons between atoms. Hydrogen (H2) – which does not occur in nature – can be split out of water (H2O) leaving oxygen. In ammonia; NH3, each atom of nitrogen holds three hydrogen atoms.
PV electrolysis is already available commercially
To chemically rearrange these molecules, readily available electrolysis can be supplied by electricity from any renewable energy source already.
“You can walk up to Siemens for example, and you can buy a multi-megawatt electrolyzer straight away. It’s commercially available, so you can just put that onto a PV farm. You can do that tomorrow morning,” said Lovegrove.
In any region globally where renewable electricity costs are $30 USD per MWh or less, solar or wind electrolysis would be competitive with natural gas-based ammonia production, which emits 1.7 tons of CO2 per ton and costs between $200 and $600 per ton, according to the IEA: Renewable Energy for Industry.
CSP could cut costs nearly in half
Heat drives the current fossil-fuel-based Haber-Bosch process discovered in 1909. Purified nitrogen and hydrogen gas is heated to 400° to 650° C (emitting CO2) at pressures ranging from 200 to 400 atmospheres, after separating hydrogen from natural gas – in a process that also emits CO2. Fossil-based ammonia manufacturing already emits 1% of global greenhouse gases – just to supply fertilizer demand. Efficient solar production is essential if NH3 is to be an energy carrier.
But heat at 800 C to over 1000 C can be supplied in solar reactors. CSP’s more efficient water splitting promises to halve the cost of hydrogen production from solar electrolysis, because in concentrating and reflecting solar flux from heliostats (mirrors) into a reactor, heat is made directly.
While splitting water in solar reactors is already being researched, the nitrogen reaction needed for ammonia manufacturing is a new research field that has just begun at Germany’s DLR, which is affiliated with IEA’s SolarPACES.
Ammonia as a fuel has received sporadic attention for over a century, but with today’s advances in solar fuel research, NH3 holds promise for a new era of solar energy traded globally as liquid fuel.
_____________________________________
More reading:
Design and optimization of an ammonia synthesis system for ammonia-based solar thermochemical energy storage
Solar Energy, Volume 159, 1 January 2018, Pages 992-1002
Chen Chen, Keith M. Lovegrove, Abdon Sepulveda, Adrienne S. Lavine
https://doi.org/10.1016/j.
Modeling of ammonia synthesis to produce supercritical steam for solar thermochemical energy storage
Solar Energy, Volume 155, October 2017, Pages 363-371
Chen Chen, Hamarz Aryafar, Keith M. Lovegrove, Adrienne S. Lavine
https://doi.org/10.1016/j.