Abstract:
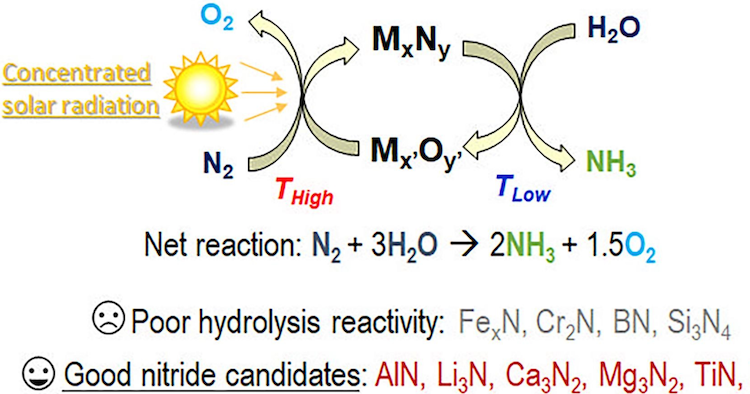
Ammonia is a fundamental chemical commodity for fertilizers and as a novel energy vector. Solar-driven ammonia synthesis is proposed as a sustainable alternative to the catalytic energy-intensive and CO2-emitting Haber-Bosch process. The considered thermochemical process aims to produce ammonia from nitrogen and water (N2 + 3H2O → 2NH3 + 1.5O2) via redox cycles using a solar heat source, thus bypassing the supply of H2 or electricity. Metal oxide/nitride redox pairs can be employed for this cyclic process. The exothermal hydrolysis reaction of nitrides produces ammonia (MxNy + 3H2O → 2NH3 + Mx’Oy’), and is followed by one or several regeneration steps (Mx’Oy’+N2 → MxNy+3/2O2) requiring a heat supply from concentrated solar energy. This study aims to experimentally identify the most suitable metal nitrides in the hydrolysis step for ammonia synthesis based on solar-driven chemical-looping. As a result, FeN, CrN, BN, and Si3N4 turned out to be irrelevant candidates for NH3 production, as the hydrolysis yield was poor up to 1000 °C. In contrast, AlN, Li3N, Ca3N2, Mg3N2, TiN, and ZrN exhibited noteworthy reactivity depending on the temperature. The hydrolysis rate of AlN was significantly enhanced only above 1100 °C, TiN showed an increasing NH3 production rate with temperature (reaching 3.4 mmol/min/g at 1000 °C), while an optimum at 750 °C was unveiled for complete ZrN conversion (corresponding to the highest rate of 34.2 mmol/min/g). Hydrolysis of Li3N, Ca3N2, and Mg3N2 was complete at lower temperatures (∼200 °C), with NH3 yields of 5.9, 4.9, and 18.6 mmol/g, respectively. Solar-driven regeneration of metal nitrides at high temperature will be then necessary to demonstrate the complete feasibility of thermochemical cycles for green ammonia synthesis.
Abanades, S., Rebiere, B., Drobek, M., & Julbe, A. (2024). Experimental screening of metal nitrides hydrolysis for green ammonia synthesis via solar thermochemical looping. Chemical Engineering Science, 283, 119406. https://doi.org/10.1016/j.ces.2023.119406